��ӭ������52ijӢ��������������С��Ϊ�������Ӣ��֪ʶ�ǣ���ƻ���ֻ��Ծɻ��� ԭ��������������������������ϸ�ķ�����
ƻ���ֻ��Ծɻ��� ԭ��������������

С�ർ����3��31�գ�ƻ�����Ծɻ��¼ƻ���ʽ���й���½ִ�С�������6������ƻ���ֻ������Բ��롣���а���4�Ļ��ռ���250Ԫ��4S500�飬5S����1500�������������²ۻ��ռۡ�̫�͡���������������ţ��ͬʱ�������û�������Ϊʲô�ֻ�Ҫ�Ծɻ����أ�
An average smartphone contains 41 different elements - and last year alone 1.2 billion of the handsets were sold globally.һ���ֻ���һ�������41��Ԫ�أ�ȥ��һ��ȫ���������12�ڲ��ֻ���
This is causing an imbalance between the supply of metals and metalloids and their demand from consumers - a concept referred to as 'criticality.'�����ᵼ�½�����������Ĺ�Ӧ������������֮��IJ�ƽ�⣬����Ϊ���ٽ�״̬����
Researchers have now assessed the criticality of all 62 metallic-based elements on the Periodic Table to reveal which are most at risk and what that means for our gadgets in the long-term. �о���Ա���Ѷ�Ԫ�����ڱ��е�62�ֽ���Ԫ�ص��ٽ�״̬�������������г��˴��ڱ�Σ״̬�Ľ������������ӳ�Զ�Ƕȷ�������Щ�����Ĵ����ܷ�������������С���ߵ�����
Metal criticality is analysed in three ways - supply risk, vulnerability to supply restriction (VSR) and environmental implications. �������ٽ�״̬ͨ�����ַ��������ó�����ӦΣ������Ӧ��Լ�Ĵ����ԣ�VSR���Լ�����Ӱ�졣
Using these three categories, researchers from Yale University led by Professor Thomas Graedel were able to see which metals and metalloids are most at risk within each group. ͨ��ʹ����������ָ�꣬����˹•���¶����ڵ�����Ү³��ѧ���о���Ա�ó��˴��ڱ�Σ״̬�Ľ���������
Many of the metals traditionally used in manufacturing, including zinc, copper, and aluminum, are not at risk, explained Professor Graedel. ���¶�����˵�������ڴ�ͳ����ҵ��ʹ�õ��Ľ�����п��ͭ�����Ȳ�δ���ڱ�Σ״̬��
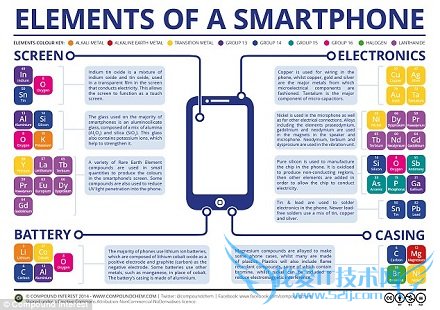
However, the newer or less-common metals used in smartphones, infrared optics, and medical imaging, are vulnerable.��������Խ��µ������ֻ�������⾵��ҽѧ�����������ʹ�õ��ġ���Բ�������ϡ�н��������Ƚ�Σ�ա�
The team's results show that the limitations for many important metals used in smartphones and other gadgets are largely those related to supply risk.���Ŷӵ��о������ʾ�����ֻ�������С��е��ʹ�õ���������Ҫ����������Ϊ��ӦΣ�����ܵ����ơ�
These include gallium used in processor chips and selenium in transistors on these chips. ��Щ�����������ݴ���оƬ��ʹ�õ������Լ���ЩоƬ�ľ������ʹ�õ�������
Platinum group metals including gold and mercury have the highest environmental implications. �������𡢹����ڵIJ�������Ի�����ɵ�Σ�����
'The metals we've been using for a long time probably won't present much of a challenge,' said Professor Graedel. ���¶�����˵�������Ƕ��Ѿ�ʹ���˺ܳ�ʱ��Ľ����������õ��ġ���
'We've been using them for a long time because they're pretty abundant and they are generally widespread geographically. ������������ô��ʱ�������Ϊ���Ǵ����ḻ�������Ϸֲ���Ҳ�ܹ㡣��
'But some metals that have become deployed for technology only in the last 10 or 20 years are available almost entirely as byproducts. ���������һ��ʮ������ĿƼ���ҵ����ʹ�õĽ�����������Ϊ����Ʒ���ֵġ���
'You can't mine specifically for them; they often exist in small quantities and are used for specialty purposes. And they don't have any decent substitutes.' ����������ȷ��ֱ�Ӷ���Щ�������п��ɣ����Ǵ���С����;�ض�������û�к��ʵ����Ʒ����
The researchers listed these as indium, arsenic, thallium, antimony, silver, and selenium. There is no substitute for indium, in particular, and this could impact our touchscreens. �о���Ա�г�������������������������飬�裬��������������û���κο���������Ľ�����������������������Ҫ�õ�����Ҫ����֮һ��
- �����б����������۽������ѱ�����˿���������������վͬ����۵��֤ʵ��������
-
