欢迎您访问52IJ教育培训网,今天小编为你分享的高考英语方面的学习知识是通过网络精心收集整理的:“己烷_环己烷椅式构象比船式稳定的原因是什么环己烷为什么...[英语]”,注意:所整理内容不代表本站观点,如你有补充或疑问请在正文下方的评论处发表。下面是详细内容。
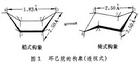
按照碳原子具有正四面体构型的学说,环己烷分子中的六个碳原子在键角(109.5°)保持不变的情况下,可以两种不同的空间形式,组成六元环,称为环己烷的船式构象和椅式构象 (图1).按照碳原子具有正四面体构型的学说,环己烷分子中的六个碳原子在键角(109.5°)保持不变的情况下,可以两种不同的空间形式,组成六元环,称为环己烷的船式构象和椅式构象 (图1).
根据现代分子结构理论,由于基团的相互作用
的缘故,椅式构象比船式构象稳定得多,常温下环己烷几乎完全是椅式构象.
通过船式构象的纽曼投影式(图2),
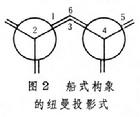
可以看到碳原子1、2、4、5上相连的氢原子都处在全重叠式的位置上.从船式构象的透视式可以看到碳原子3和6(或称船头和船尾碳原子)上的两个向环内伸展的氢原子相距较近.上述两种情况都使氢原子之间产生较大的斥力,从而产生一种使船式构象扭转为椅式构象的内在力量,这种力称为扭转张力.这是船式构象不稳定的根本原因.在椅式构象中,组成碳环的任何相邻的两个碳原子上的氢,彼此都处在交叉式的位置上(图3),
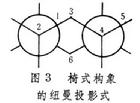
它们之间无扭转张力,比较稳定.
英文版解释:
Conformation[edit]
Main article: Cyclohexane conformation
The 6-vertexed ring does not conform to the shape of a perfect hexagon.The conformation of a flat 2D planar hexagon has considerable angle strain because its bonds are not 109.5 degrees; the torsional strain would also be considerable because all of the bonds would be eclipsed bonds.Therefore,to reduce torsional strain,cyclohexane adopts a three-dimensional structure known as the chair conformation.There are also two other intermediate conformers; half chair,which is the most unstable conformer,and twist boat,which is more stable than the boat conformer.This was first proposed as early as 1890 by Hermann Sachse,but only gained widespread acceptance much later.The new conformation puts the carbons at an angle of 109.5°.Half of the hydrogens are in the plane of the ring (equatorial) while the other half are perpendicular to the plane (axial).This conformation allows for the most stable structure of cyclohexane.Another conformation of cyclohexane exists,known as boat conformation,but it interconverts to the slightly more stable chair formation.If cyclohexane is mono-substituted with a large substituent,then the substituent will most likely be found attached in an equatorial position,as this is the slightly more stable conformation.
Cyclohexane has the lowest angle and torsional strain of all the cycloalkanes,as a result cyclohexane has been deemed a 0 in total ring strain,a combination of angle and torsional strain.This also makes cyclohexane the most stable of the cycloalkanes and therefore will produce the least amount of heat (per CH2 unit) when burned compared to the other cycloalkanes.
其他类似问题
问题1:如何快速画好环己烷椅型构象RT,本人手笨,画不好.
先画上下两条平行线,等长,但要错开一半,然后画一上一下两个角,对应的键保持平行就可以,多练习就可以了.
问题2:甲基环己烷有几个构象异构体?(考虑船式和椅式)其中那个最稳定,那个最不稳定?为什么?[化学科目]
考虑到船式、椅式、a键(竖直键)、e键(水平键),应该有4(=2×2)个构想异构体.椅式+e键最稳定(椅式比船式稳定,此外e键比a键稳定);船式+a键最不稳定.
问题3:谁有环己烷的椅型构象详图?
就是这个...刚手画的...也不知道你要详到什么程度的,给你最简单的...

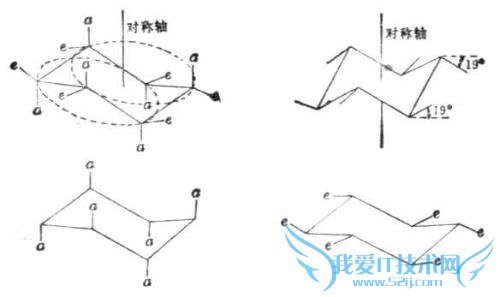
问题4:环己烷构象,为什么H在e键上比a键稳定[化学科目]
因为e键上的氢离周围的氢更远一些(他们都是朝外的)所以他们的能量更低.这个问题我当初也和化学老师提过,他让我亲手搭了一个球棍模型,一看就发现了.我找到一张图你看一下吧.
问题5:为什么顺-1.3-二甲基环己烷的顺式构象比反式构象更稳定[化学科目]
一般来说取代基尽量取平键,以避免位阻效应.对于有顺式1,3-双取代的分子,两取代基还要避免1,3-双直键立体作用.
- 52bus_...sartion,you can _49_ the NO.9 bus to
- 宝马狂想曲下载_宝马狂想曲中bus metro[英语]
- 清扬的意思_optically clear是什么意思[英语]
- it's over音译歌词_it`s all over but the crying
- 电子邮件怎么写_英语邮件怎么写?[英语]
- 11月12日是什么节日_...?60岁10.今天是几月几号
- imagine的用法_imagine的用法,imagine sb doing
- 国庆庆典邀请函_写一篇英文80字 国庆节邀请信 邀
- 谓语是什么_we have arrived谓语是什么[英语]
- forever stand by you_Forever stand dy you rlop
- 评论列表(网友评论仅供网友表达个人看法,并不表明本站同意其观点或证实其描述)
-
